Target RWE at EASL 2025
Learn how we advance liver disease research through collaboration and evidence generation
Explore Our Liver Disease Research at EASL
Target RWE attended the EASL Congress 2025 from May 7-10th in Amsterdam. We showcased two new posters highlighting our latest liver disease research and insights on the role of RWE in advancing treatment and patient care. EASL 2025 was fantastic opportunity to connect with thought leaders, explore cutting-edge research, and collaborate on shaping the future of hepatology.
Activity at EASL 2025

PBC
Consensus conference: surrogate end-points and real world evidence in Primary Biliary Cholangitis (PBC)
Featuring Michael W. Fried, MD, FAASLD, Co-Founder & Chief Medical Officer, Target RWE
The EASL Consensus Conference on PBC Therapies brought together leading experts in primary biliary cholangitis to address critical questions surrounding the evolving treatment landscape. The conference aimed to establish consensus on key aspects of PBC management, including the role of current and emerging therapies, patient stratification, and optimal treatment algorithms.
MASH
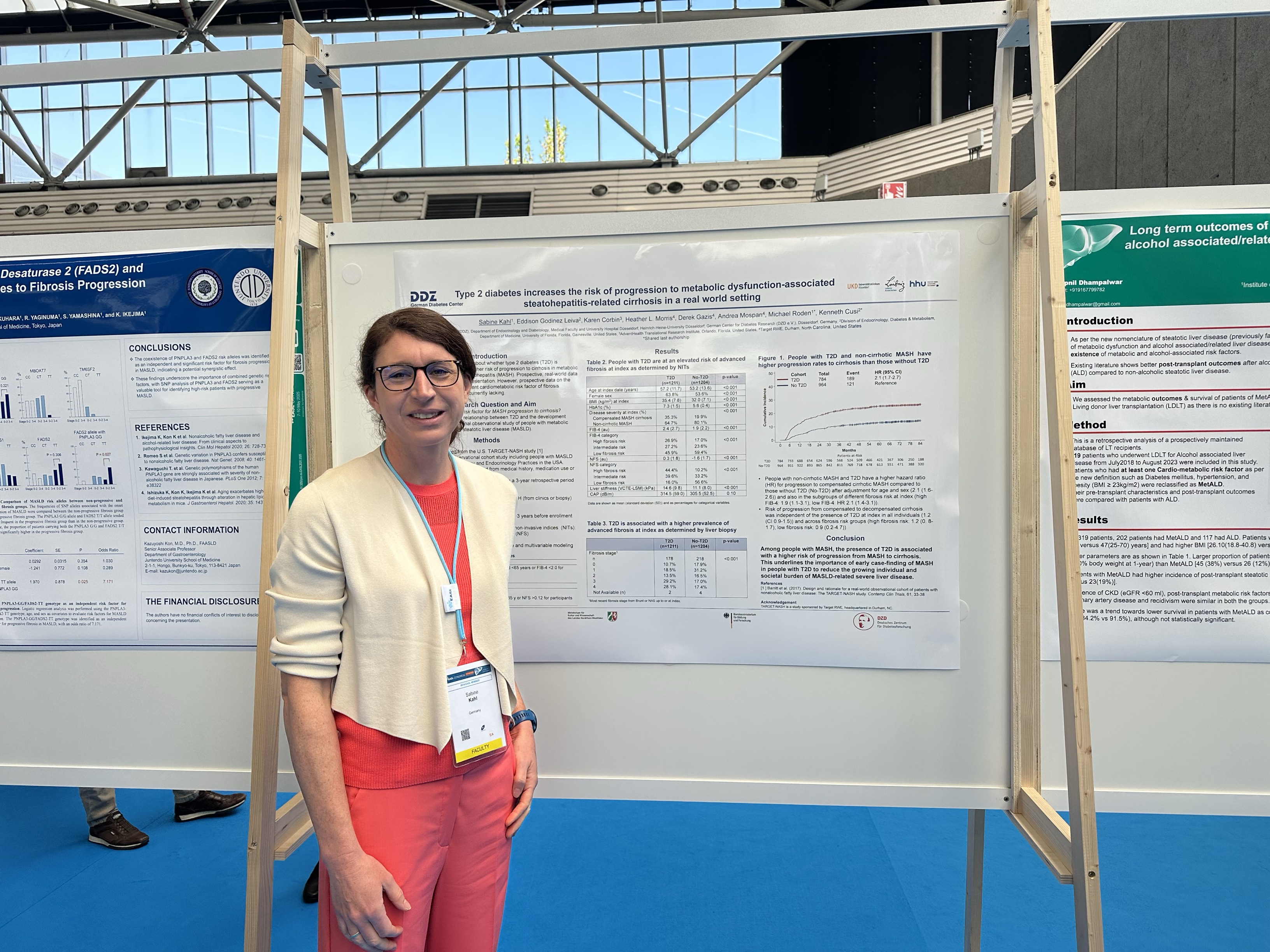
Type 2 diabetes increases the risk of progression to metabolic dysfunction-associated steatohepatitis-related cirrhosis in a real world setting.
Presented at EASL, this research utilized a longitudinal observational cohort to investigate the influence of type 2 diabetes on MASH progression. The results revealed a clear association between T2D and an elevated risk of progressing to cirrhosis, underscoring the critical role of real-world evidence in understanding disease trajectories and informing screening practices in MASLD.
HCC
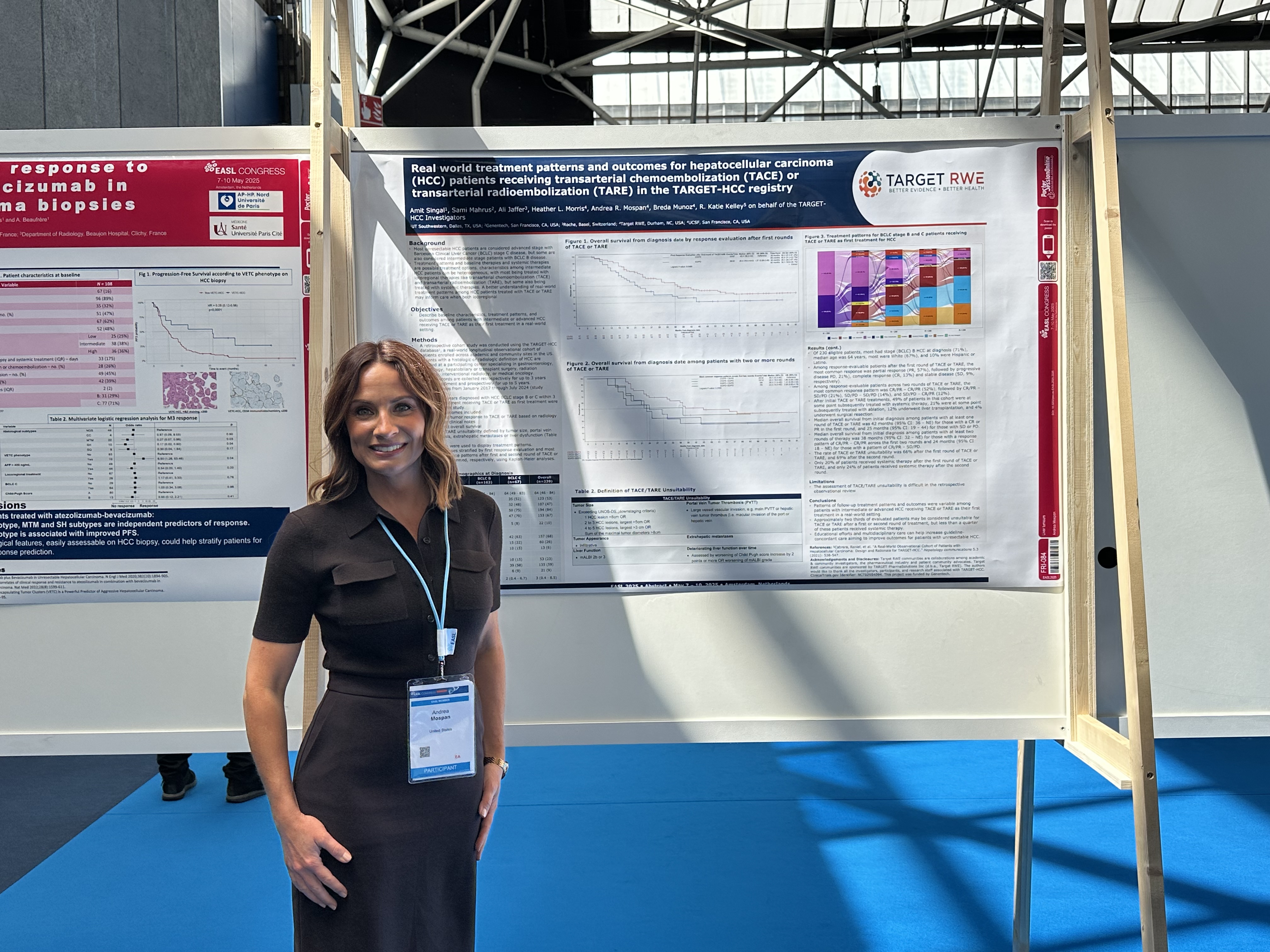
Real world treatment patterns and outcomes for hepatocellular carcinoma (HCC) patients receiving transarterial chemoembolization (TACE) or transarterial radioembolization (TARE) in the TARGET-HCC registry.
This EASL poster presentation offered valuable real-world insights into the treatment of intermediate and advanced HCC with TACE or TARE. The findings, derived from the Target RWE’s HCC patient cohort, revealed heterogeneous treatment patterns and outcomes, highlighting a potential gap in guideline-concordant care. Notably, a significant proportion of patients deemed unsuitable for further TACE/TARE did not receive subsequent systemic therapy, suggesting an area for improvement in multidisciplinary management and educational efforts within the hepatology community to optimize outcomes for patients with unresectable HCC.
Our Leadership & Expertise in Liver Disease Research

Notable Accomplishments:
- Liver Disease Cohort: Surpassed 600,000 enrolled patients, strengthening our partnership with the American Association for the Study of Liver Diseases (AASLD) and the Cirrhosis Quality Collaborative (CQC) initiative.
- Leadership in Real-World Evidence (RWE): Contributed to over 35 publications, including 5 peer-reviewed articles and 34 abstract posters and oral presentations at major medical conferences.
- Liver Meeting 2024 Highlights: Dr. Sidney Barritt presented new MASH research, showing GLP-1 RA use in MASLD is linked to slower disease progression and reduced all-cause mortality.
- Expansion of RWE Capabilities: Strengthened our ability to collect, curate, and analyze real-world data across key therapeutic areas, supporting the entire drug development lifecycle.
Presentations at EASL 2024
Extensive Patient Population & Diverse Data Collection in Liver Disease
Target RWE offers a transformative solution to address the critical evidence gaps that often define the success or failure of liver disease drug development. By leveraging our in-house real-world data (RWD) and rigorous epidemiological methods, Target RWE provides deep insights into patient populations, disease progression, comorbidities, and treatment responses, without the need for invasive biopsies or costly study designs.
This allows you to optimize clinical trial designs, reduce screen failure rates, and make faster, more informed decisions, ultimately accelerating timelines and minimizing the risk of costly delays or regulatory setbacks. With Target RWE, you can bridge the evidence gaps that are so often the difference between success and failure, ensuring your drug candidates meet the high expectations of regulators, clinicians, and patients alike.
Proud Partners with the American Association for the Study of Liver Disease (AASLD)

The collaboration between Target RWE and AASLD aims to address the unmet need for a large, real-world registry of chronic liver disease patients. This partnership enables sites to explore treatment variations, outcomes, and patient-reported measures across a broad range of liver diseases, including NASH, HBV, cirrhosis, and more.
Target RWE is rooted in longitudinal clinical care and prospective studies, leveraging real-world clinical data to drive evidence-based decisions. Participating sites will also contribute to the AASLD Cirrhosis Quality Collaborative (CQC Registry), which collects and curates data to identify variations in care and patient-reported outcomes in liver disease treatment.
Dr. Grace L. Su, 2025 AASLD President, emphasizes the collaborative benefits of CQC sites: “These sites will gain access to invaluable data resources while advancing their own research and contributing to the broader hepatology community.”